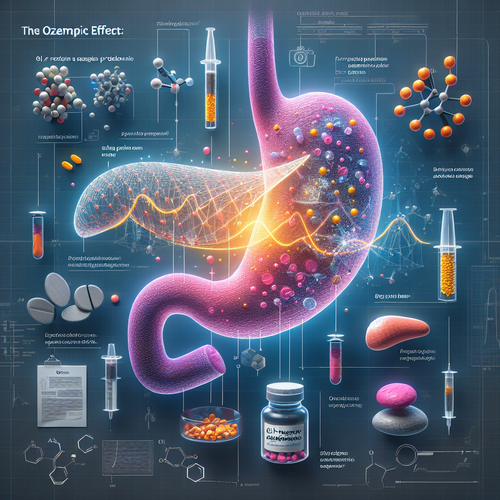
In recent years, a new class of drugs has swept across the globe, promising not just better diabetes management but also significant weight loss results that many have long sought. Medications like Ozempic, Wegovy, and Mounjaro, based on GLP-1 receptor agonists, have become household names, often discussed on social media and in news headlines for their transformative effects. For millions struggling with obesity or type 2 diabetes, these drugs have offered a glimmer of hope, potentially reducing risks associated with these conditions and improving quality of life. The sheer demand has, at times, even outstripped supply, highlighting the profound impact these pharmaceuticals are having on the health landscape. However, as with any powerful medication, the widespread adoption and longer-term use are beginning to reveal potential complications, prompting a closer look at the balance between revolutionary benefits and emerging risks. The latest concerns center on a vital, often-overlooked organ: the pancreas.
Alarming reports are surfacing, linking the use of these popular weight-loss and diabetes injectables to serious pancreas problems, specifically acute pancreatitis. This condition, characterized by sudden inflammation of the pancreas, can range from severely painful but temporary episodes to life-threatening complications, including organ failure and, tragically, death. Regulatory bodies are now taking these reports seriously. In the UK, the Medicines and Healthcare Products Regulatory Agency (MHRA) has launched a formal investigation following hundreds of reported cases. While some cases may resolve relatively quickly, the sheer volume of complaints, including some documented fatalities, paints a concerning picture that warrants urgent scrutiny. The scale of these reports underscores the need for vigilance among both prescribing physicians and patients currently using or considering these treatments.
The core of the regulatory investigation lies in understanding the precise nature of this link. Are these GLP-1 drugs directly causing pancreatic inflammation in certain individuals? Or are there other contributing factors at play? One line of inquiry being pursued by the MHRA involves exploring potential genetic predispositions that might make some patients more vulnerable to this side effect. This is a critical avenue, as identifying genetic markers could help stratify risk and ensure these powerful drugs are prescribed more safely in the future. Compounding the complexity, rapid weight loss itself, regardless of the method, can inadvertently increase the risk of gallstones, which are a known common cause of acute pancreatitis. This raises questions about whether some reported cases are a direct drug effect, an indirect consequence of successful, rapid weight loss facilitated by the drug, or perhaps a combination of both. It’s also important to note that while pancreatitis is a concern, studies so far have generally failed to establish a definitive link between GLP-1 use and pancreatic cancer, a much rarer but devastating complication, although uncertainty remains regarding long-term risks.
This emerging situation presents a significant dilemma for the medical community and millions of patients. On one hand, the benefits of GLP-1 drugs for weight management and glycemic control are well-documented and life-changing for many. They offer hope where previous interventions have failed. On the other hand, the potential for serious adverse events like acute pancreatitis, however rare in the overall user population, cannot be ignored, especially given the drug class’s increasing popularity. It highlights the perpetual challenge in medicine: balancing the known benefits against the potential, sometimes initially hidden, risks of new therapies. For healthcare providers, it means rigorous patient selection, thorough discussion of potential side effects, and careful monitoring. For patients, it means being informed, recognizing potential symptoms (like severe abdominal pain), and promptly seeking medical attention if concerns arise. The booming market for these drugs also necessitates a global, coordinated effort to collect and analyze data on adverse events transparently.
Ultimately, the investigations by regulatory bodies are a necessary step to ensure the continued safe use of these valuable medications. While the vast majority of people taking GLP-1 drugs for weight loss or diabetes will likely not experience pancreatic issues, the hundreds of reported cases and associated deaths serve as a stark reminder that vigilance is paramount. Understanding the mechanisms behind these complications, identifying high-risk individuals, and clearly communicating potential dangers are crucial next steps. As research continues and more data is gathered from real-world use, our understanding of the full risk-benefit profile of GLP-1 agonists will evolve. For now, these powerful tools for metabolic health require respect, caution, and ongoing scrutiny to ensure their benefits are maximized while minimizing the potential for serious harm.